Subject Area: Biomedicine / Biochemistry
A slight turn from why – How monkeys lose weight – Your body on fat – Sirtuins for the masses – Are you a mouse?
A slight turn from why
There is a food problem in the world, particularly in the West, one our ancestors would find ridiculous – We eat too much (and we eat terrible foods). Fats, sugar, and salt are heavily ladened in our meals, and they can be hard to get rid of if you are faint-hearted. Burgers as large as my head are served at restaurants; many eat it routinely and savor it. And guess what, numbers don’t lie. For example, 1 in 3 American adults is prediabetes. And the problem here is not a problem of the West alone, as the World Health Organization has indicated that the prevalence of diabetes has been rising more rapidly in low- and middle-income countries than in high-income countries. But diabetes is just one of the many diseases. And these diseases have a main say on how well and long we live. In fact, properly viewed aging itself is a disease, as scientists like David Sinclair have defended exhaustingly.
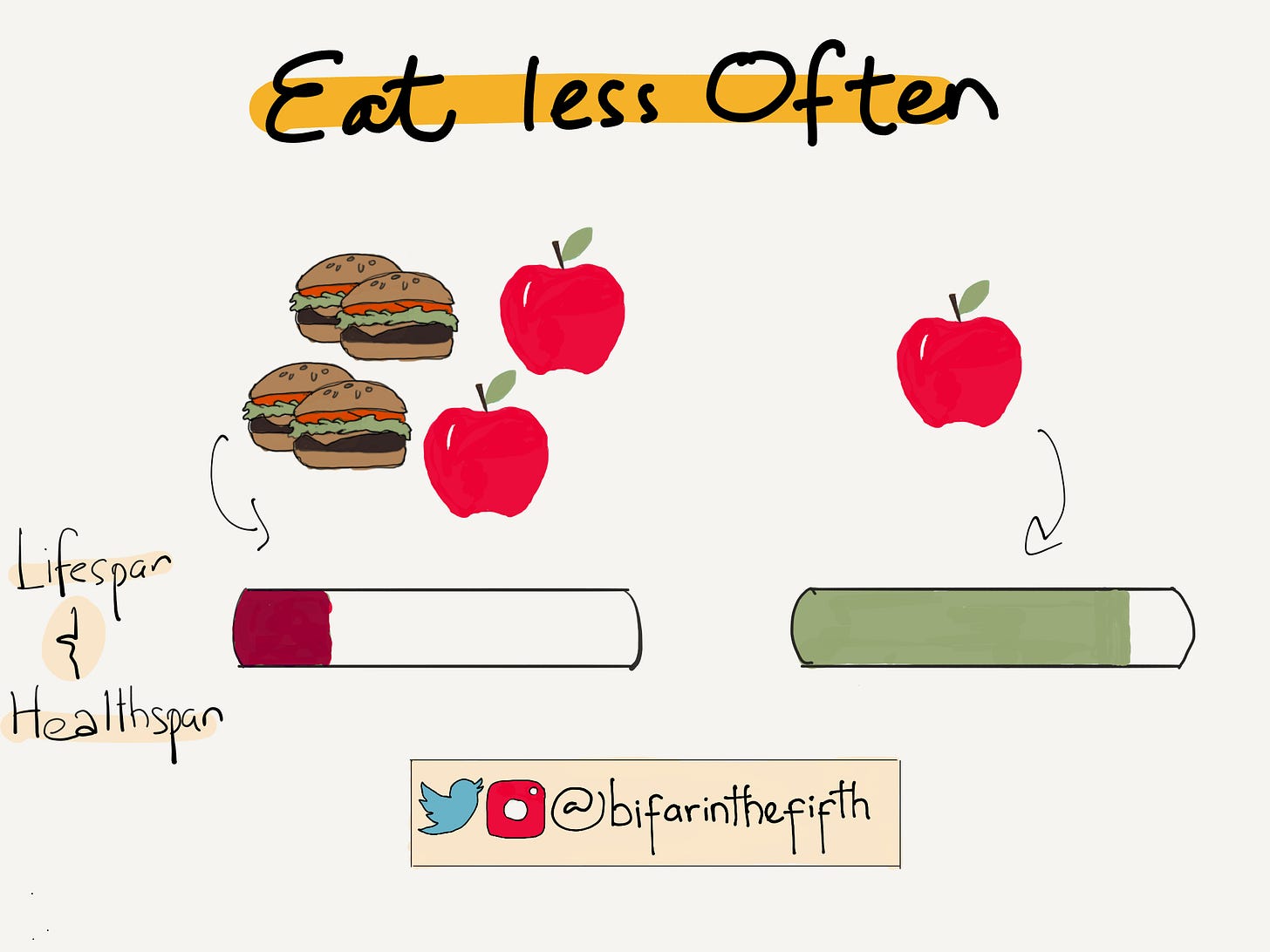
Somewhat understandably, it can be challenging for folks to do the right thing; I will wager you understand what I am saying – those apple pies taste pretty good. And running every day is just pretty damn hard. And yet the right thing has to be done; in this essay, I will write about one of the right things to do – go hungry, or at least eat less often.
Before I proceed, let me put the essay in context. This is the second installment in the series on aging I am writing. In the first installment, I discussed why we age – the biology of aging. In brief, I wrote (echoing the information theory of aging):
“When [..] genomic and epigenomic instability mounts over time, the loss of cellular identity ensues (because the accurate genetic expression for a particular kind of cell is compromised). So, genes that are supposed to be switched off go on; those that are supposed to be switched on get switched off. For example, because of the chaos in the system, the kidney cells can now begin to act up like a part brain cell, part skin cell.”
Here, I want to take a slight turn from why to how to slow aging down and where to start such discussion if not food and the absence of it?
How monkeys lose weight
Many people know this stuff, and a reminder shouldn’t hurt: we have not always had the opportunity to eat three meals a day peppered with all kinds of snacks in between. Nor have we always had the pleasure of sitting down all day in the car and in front of a computer screen. We (Homo sapiens) started off in rough ecological terrains, requiring us to go hungry for a long time, walk and run, and then repeat. It turns out that there are molecular consequences for these actions or the lack of them.
Before I go into these molecular consequences, let me start with reeling out some data.
As early as 1900, there has been an indication that caloric restriction (a dietary regimen that reduces food intake without incurring malnutrition) extends life span. For example, Osborne and coworkers in 1917 showed an increased life span in female rats who were on a restricted diet. And the data is still pilling up; in 2009, a study published in the journal Science showed that caloric restriction (CR) delays disease onset and mortality in rhesus monkeys. They reported that:
“50% of control fed animals survived as compared with 80% of the CR animals. Furthermore, CR delayed the onset of age-associated pathologies. Specifically, CR reduced the incidence of diabetes, cancer, cardiovascular disease, and brain atrophy.”
And yet, in another study on caloric restriction carried out on the smallest primate in the world, the authors reported evidence that:
“Chronic, moderate (30%) caloric restriction, when started early in adult life, can extend the lifespan of mouse lemurs by 50% and reduce the risk of age-associated diseases including cancer and chronic nephritis and age-associated mortality.”
In short, the researchers show evidence for an increase in lifespan and healthspan (period of time spent in good health).
Finally, a meta-analysis published a decade ago in an aging journal for dietary restrictions in rats and mice from 1934 to 2012 showed an increase in median life span. So, we are talking about a century's worth of solid research here.
And why does this hold up? I will turn first to some biochemistry, just a little bit.
Your body on fat
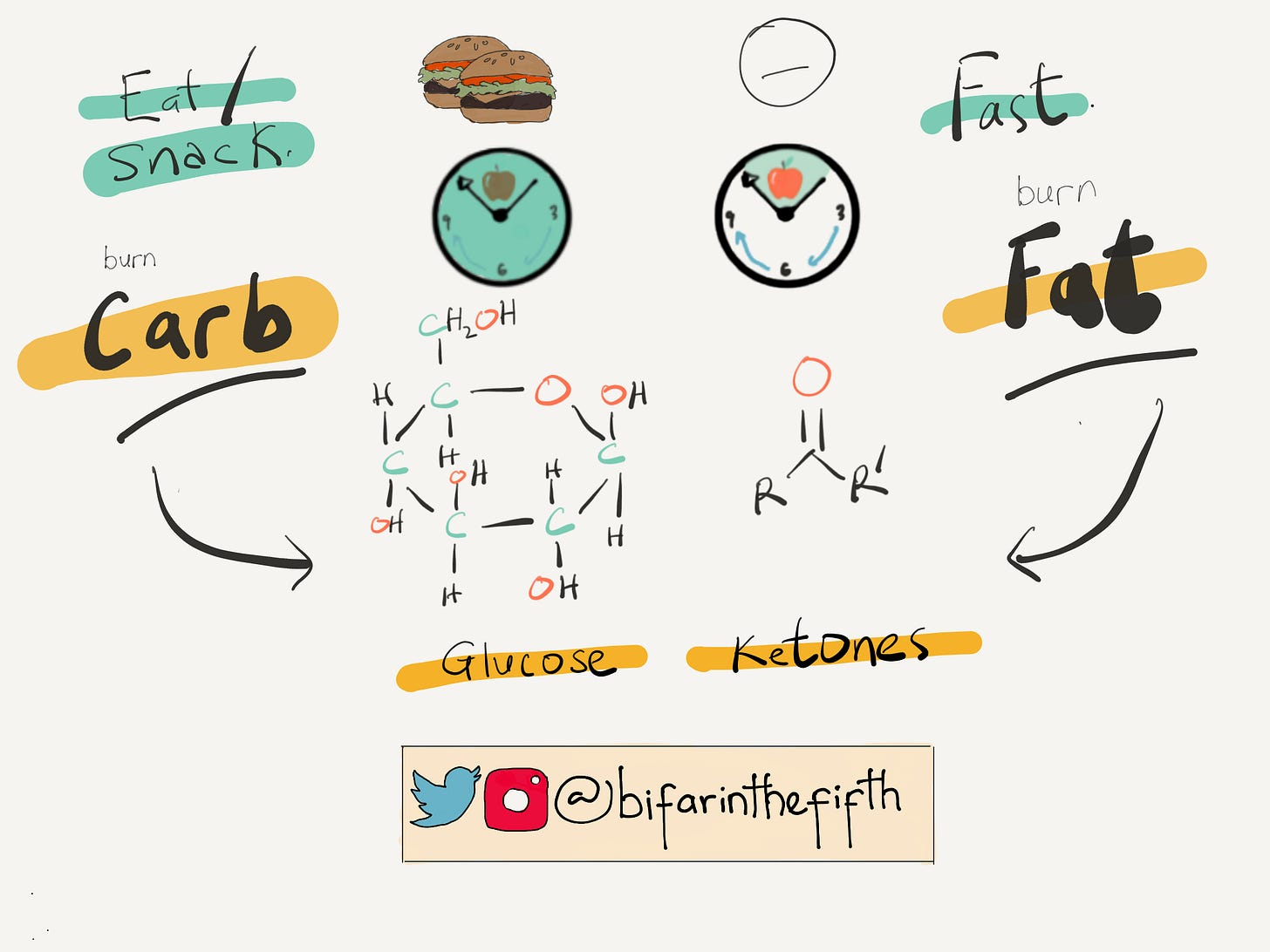
Your body has two primary energy sources, 1) (Carb) glucose and 2) fat. The primary energy source is glucose, which means glucose is used first, no matter what else is present. However, the problem is that if you take a lot of sugar without burning them, they get converted into fats (triglycerides) in adipose tissues to be used at later-day when it is needed. Adipose tissue is what you might know as body fat, which can be found all over your body (your adipose tissue helps you collect, store and release lipids.) That is why taking a lot of sugar makes you fat. There are implications for this in Diabetes, as my reader might know, but that is not the fish I want to fry in this essay, so let’s proceed.
If you now so desire to fast, which usually leads to caloric restrictions, the glycerides (the fat we met just a few sentences ago) undergo hydrolysis to give fatty acids and glycerol. Fatty acids, in turn, are then converted to ketone bodies.
It then follows that when you are well-fed, your ketone body levels are low; however, during fasting starting from 8-12 hours, ketone bodies level will be on the rise because your body needs energy, and in the absence of glucose, it turns to fat. So, it’s no wonder people lose weight when calories are restricted.
Okay, now that I have mapped out the ‘food biochemistry’ in a few sentences, my primary goal here is not the metabolism; I mean to touch on why caloric restriction and intermittent fasting make animal and primate models live longer and healthier. I turn next to more biochem and some molecular biology.
Sirtuins for the masses
There are about three longevity pathways, but I will focus on Sirtuins (silent information regulator) here. Sirtuins are classes of enzymes that regulate numerous molecular pathways, which can get complicated very quickly (its biology, after all).
Technically, we say that they are NAD+ dependent protein deacetylases. But the bottom line is that they control gene expression; that is, they turn genes on and off – in essence, Sirtuins are bloody switches. It uses a fuel called NAD, an acronym for nicotinamide adenine dinucleotide. During times of stress, such as lack of food, it is thought that ketosis (the process your body undergoes when you don’t have enough carbohydrates to burn for energy) leads to the activation of Sirtuins. And this is how it is thought to happen. If you haven’t taken a biochemistry class, proceed with caution 😊.
Cellular energy production is dependent on the metabolic coenzyme NAD+. A coenzyme is simply a compound that an enzyme requires to engage in catalysis; like I said earlier, feel free to think of it as a fuel.
Furthermore, the whole point of breaking down food and what-nots is to generate energy that can be used to do work in the cell. The energy shuttle used for this cellular operation is called adenosine triphosphate (ATP). And you can also think of ATP as cash (money). You work for a company, get paid your salary, and then use your hard-earned money to engage in economic activities.
Likewise, energy is stored in carbohydrates and fats molecules; when they are broken down and further metabolized (i.e., you working in that company), ATP is released (salary paid) and so you have some cash to spend next time you are going to the supermarket, or some coin to buy your favorite NFT – whichever suits you.
Carbohydrates get broken down into glucose while fatty acids get oxidized into ketone bodies; I guess we are already clear on that. Recall that when you are well-fed, your body uses glucose; when you fast, it’s ketones. In the cell, glucose and ketone bodies are metabolized to give ATP (money), with each pathway requiring a different amount of NAD+ – which is crucial. Following through with the analogy (since we have agreed to think of it as fuel), NAD+ is the energy you expend at your place of work to get cash (ATP)
And here is the main takeaway: For the same amount of ATP produced, glucose metabolism utilizes four times more NAD+ than ketone metabolism. That is: if you work in a ‘glucose company,’ you expend more energy than in a ‘ketone company.’
In short, it is believed that the increased level of available NAD+ while you fast (i.e., when you burn fat and metabolize ketones) is responsible for activating Sirtuins.
On the other hand, Sirtuins can delay cell death and extend lifespan by maintaining genome integrity and being involved in DNA damage repair. Recall that aging occurs due to the instability in the genome and the epigenome (see my essay why we age). So, Sirtuins help to pretty much counteract this hallmark of aging.
Now let’s attempt to put our analogy to rest: Sirtuins are like the extra motivation you get to work on your side gig when you get home from work, so you can get more money to take care of yourself. If you work in the ‘glucose company’ you are exhausted when you get home. On the other hand, if it’s in the ‘ketone company,’ you have fuel (NAD+) to kick more asses.
Are you a mouse?
A few hundred words ago, I cited some studies showing definitive evidence of caloric restrictions and an increase in lifespan. A popular critique of these studies is, “are you a mouse?” But such critique might be too hasty if one is to realize that there is a reason we have model organisms: they are great pointers, and we have learned so many beneficial science by studying them.
While caloric restriction longevity benefits have been shown in yeast, fruit flies, rodents, c elegans, dogs, mouse lemurs, rhesus monkeys, and God knows what else. As far as I can tell, its effects on human longevity are relatively unknown (in terms of solid, hard evidence) – and for good reasons: we will need a century or so to conduct such definitive experiments. However, we do know for a fact that intermittent fasting and caloric restriction improves obesity, hypertension, inflammation, insulin resistance, etc. – which are all diseases that will invariably impact lifespan and healthspan. So I guess that is all we need for now.
But we still have anecdotes and observational studies, don’t we?
Of course, we know of the blue zones – regions around the world filled with people who lived longer than the average folk. One of such is the Japanese Okinawans, who have a habit of eating healthy and follow an ancient Confucian tradition they call ‘Hari Hachu Bu,’ where they deliberately stop eating before they are full (a.k.a. caloric restriction). Another is an island where people forget to die – Ikaria, Greece. Amongst other healthy ways of living, Ikarians fast occasionally (religious rights), requiring fasting for almost half a year. In Ikaria, one in threepeople makes it to 90.
And so, what are you waiting for: Eat less often.
Recommended readings:
Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 2019;381:2541-51.
Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194.





Great piece
Such a good read. Thank you for penning this.